Eagle Air Freight’s journey to GDP-ready temperature compliance
How Eagle Air Freight conducts GDP-compliant temperature mappings and established compliance procedures fit for healthcare logistics.

Quick overview
Jump to the detailed case study.
Use case:
- Company: Eagle Air Freight
- Industry: Pharmaceutical logistics
- Company size: Approximately 40 employees
- Location: Northeastern United States
- Solution: GDP temperature validation of temperature-controlled trucks
The challenge:
Transitioning from general freight to healthcare logistics. This process included ensuring that the conditions in the logistics company’s temperature-controlled trucks complied with the requirements of GDP and that all procedures were up to the compliance standards necessary for pharmaceutical shipping.
The solution:
Partnering with Eupry to review practices and procedures and conduct thermal mappings of the fleet to make sure everything aligns with GDP standards and meets the requirements of pharmaceutical products.
The outcome:
Enhanced operational standards and a competitive advantage in the pharmaceutical logistics market.
Primary reasons for choosing Eupry:
- Strong reputation
- Validation competence
- GDP expertise
Also see: How Eupry's mapping solutions work

About Eagle Air Freight
Eagle Air Freight, based in the Northeastern United States, provides pick-up and delivery services across New England and linehaul services to and from New York and New Jersey. The logistics firm collaborates with major U.S. airlines and handles ocean container work, regularly consolidating freight at cargo stations in New Jersey. Initially focusing on general cargo, the company has now shifted its dedicated focus to healthcare logistics, serving the region's hospitals, universities, and pharmaceutical firms.
What would you tell a peer about Eupry’s mapping and validation solutions?
Describing my experience with Eupry, the qualities that stand out to me are reliability, transparency, and service. As someone new to the pharmaceutical logistics industry seeking guidance, I found the direction and clarity I needed with the Eupry team and built confidence that all protocols and standards were correctly implemented. Unlike others who might promise similar services, Eupry operates with integrity without cutting corners, which is especially crucial in our industry where adherence to standards is required
Mike O’Brien, President of Eagle Air Freight
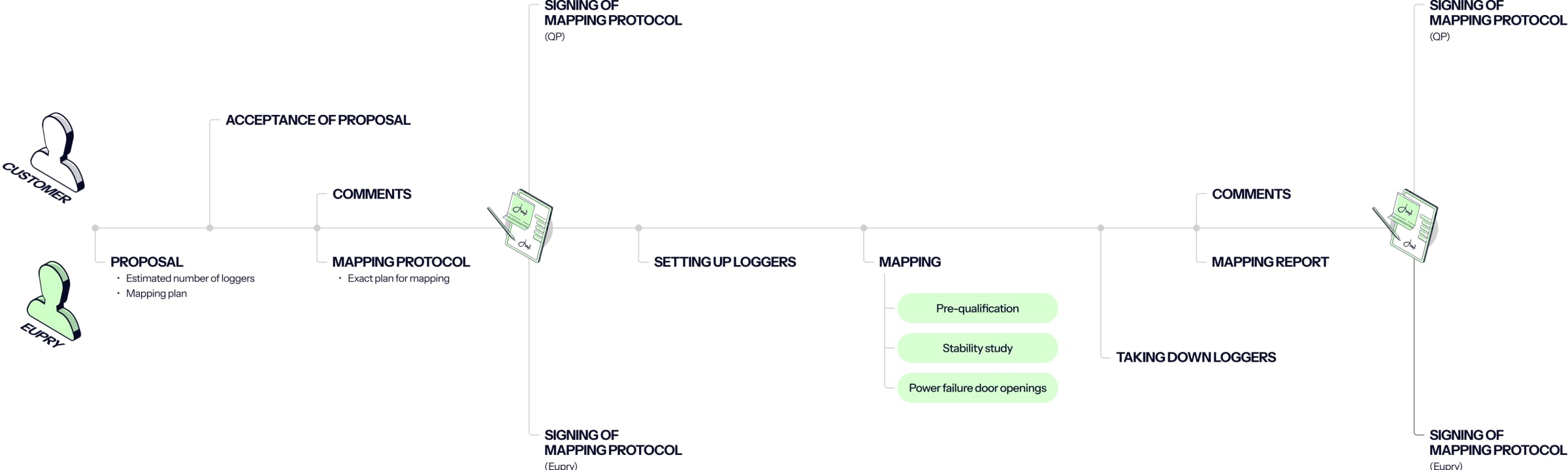
How Eupry's mapping solutions for GxP work
Eupry offers different solutions tailored to fit the temperature compliance needs of GDP and GMP companies.
Learn more about the mapping solutions.
** At a high level, the temperature mapping and validation solutions can be summarized as follows:**
Temperature mapping kit
Wi-Fi-based data loggers, specialized software, and more. One kit, all the GxP-compliant equipment you need for your study.
Remote mapping service
Eupry’s validation team takes care of all planning and reporting, and you simply set up the loggers according to the agreed plan.
On-site mapping service
The Eupry team handles everything – planning, execution, and reporting. This is the solution Eagle Air Freight went with in the end.
The more detailed version
In this case study, we dive into how Eagle Air Freight made sure their fleet and temperature compliance practices were up to par as they transitioned from general freight to the heavier regulated pharmaceutical logistics field.
1. The challenge: Securing a pharma-level compliant logistics operation
In this case study, we dive into how Eagle Air Freight made sure their fleet and temperature compliance practices were up to par as they transitioned from general freight to the heavier regulated healthcare logistics field.
The challenge: Securing a pharma-level compliant logistics operation
Eagle Air Freight faced the challenge of distinguishing itself in a highly competitive freight market where businesses often significantly undercut prices to win business. This “race to the bottom”, focusing on low prices and subsequent lower-quality services, clashed with the company's commitment to delivering consistently premium services.
This led to the decision to pivot towards healthcare logistics, an industry that matched Eagle Air Freight’s wish to focus on high quality all around.
Why the shift to healthcare logistics?
Eagle Air Freight’s commitment to delivering quality service was a significant driver behind the shift to healthcare logistics, where high quality is a key component.
Mike O’Brien, President of Eagle Air Freight, explains:
In the general freight market, it is common to drastically undercut prices and then, consequently, deliver an ‘undercut’ service. However, this matched very badly with what we wanted to offer. We treat every exchange as a premium service, which doesn't align with the low-bidding nature of general freight. We ensure our drivers are well-compensated, we maintain top-notch equipment, and we own the facilities we operate from – and we didn’t want to change any of that. This commitment to quality throughout our operation was a key factor in our decision to switch to healthcare logistics, where the demand for quality service matches our offerings. The shift allows us to avoid competing with less reliable carriers and establish ourselves firmly in a market that values quality.
Getting ready for healthcare logistics
Even though Eagle Air Freight already focused on high quality in all aspects of the operation, the shift required them to meet the new stringent requirements of their new clientele, which included pharmaceutical manufacturers, hospitals, universities, and similar.
To establish trust and gain a competitive edge, they therefore needed to:
- Ensure that their operations and fleet were equipped to safely handle sensitive products.
- Develop procedures that comply with the strict regulatory standards associated with pharmaceutical and life science products.

2. The solution: GDP-compliant validation
Introducing GDP and WHO guidelines
A large part of the shift to healthcare logistics was understanding and implementing the new regulations and guidelines that characterize the pharmaceutical industry.
What needed to change when you shifted from running general freight into a healthcare logistics operation?
Because it was a new area for us, it was a little bit of everything. We needed to get all of our operating procedures and protocols in play and determine exactly what we needed to be compliant. It really forced us to go back and review our roots and almost start fresh, basing our whole operations on GDP and WHO guidelines. But it was definitely for the better and made us a better company,
Mike O’Brien explains.
Also read: What are the WHO’s guidelines for temperature mapping?
For Eagle Air Freight, an important step towards succeeding in pharmaceutical logistics was obtaining GDP (Good Distribution Practices) certification. During this process, Mike O’Brien also experienced the big difference between the requirements of the traditional trucking industry and the requirements of healthcare logistics regarding documentation and SOPs.
In short, documentation is everything.
As we have been going through this process, I found that Europe*, in the form of GDP, is much more advanced when it comes to the supply chain of pharmaceuticals than has traditionally been the case in the United States. It has been clear that assumptions are a big no in any GDP-compliant SOP. In much of the traditional trucking industry, procedures tend to be assumed. For instance, who orders additional data loggers, and who handles it if that person is unavailable? Saying, ‘Well, I am always here’ is not adequate. It must be documented. ‘If Mike is out, this specific person steps in,’ and this must be clearly defined in their job description as well, Mike O’Brien says.
*GDP is traditionally a European guideline but has increasingly been adopted in the US over recent years, not the least due to the FDA’s adoption of the guidelines.
Also watch: The 6 steps to navigating the FDA’s adoption of GDP guidelines

Next up: GDP-level thermal validation of truck fleet
Eagle Air Freight's strategic shift extended beyond simply understanding requirements; it was about actively integrating these into their daily operations and procedures to ensure they consistently met the standards.
A large part of this work was securing the fleet’s ability to handle temperature-sensitive products securely and live up to GDP guidelines — and here, temperature validation and mapping* was a key requirement.
- Note: Temperature mapping is the process of systematically recording and analyzing temperatures throughout a specific storage area to ensure conditions meet required standards.
How to choose a thermal validation and mapping provider?
Previously, Eagle Air Freight used their temperature-controlled trucks for shipping perishable items that, unlike pharmaceutical products, did not require mapping, and therefore, they now had to determine the most suitable method for conducting these necessary studies.
When we decided to transition from the perishable side, we had never done a mapping study or anything like that because perishable goods are a very unregulated industry compared to life science, Mike O’Brien explains.
To define and initiate a validation process where Eagle Air Freight could feel secure that it and all temperature-controlled trucks complied with pharmaceutical transport standards and GDP, Mike O’Brien, therefore, contacted the temperature compliance specialists at Eupry.
Why did you choose Eupry?
We chose Eupry’s mapping solution and services because of their strong reputation and positive reviews. At the time, there was no clear standard for healthcare logistics validation in the United States. People had an expectation, but no standard had really been established yet. So, we needed a reliable partner who could guide us through the process, Mike O’Brien says.
Planning the mapping: Establishing the baseline
Since the first interaction, Eupry has more than lived up to the expectations:
I connected with Mads, a product specialist at Eupry, and told him that we were missing a thorough understanding of the requirements of the pharmaceutical supply chain because it was all so new. We started working together, and it was great because he really helped us understand and set up exactly what we needed to get a solid baseline for our operations.
Conducting the mapping: From DIY to full on-site support
When it came to carrying out the mapping studies, Eagle Air Freight first opted for one of Eupry’s “do it yourself” solutions. They rented all the necessary data loggers and other mapping equipment, got access to specialized mapping software, and received a custom protocol from the Eupry team. In the end, the goal was to get everything they needed to carry out the studies themselves.
Initially, Mads from Eupry introduced me to the entire mapping process, starting with a 3D rendering of our trailers to decide where to place the data loggers and how to execute the study, all with the aim of conducting it ourselves, says Mike O’Brien.
Later in the process, Eagle Air Freight transitioned into getting hands-on support for Eupry’s validation team and bringing one of their specialists on-site to carry out the studies.
The mapping software was a game-changer for me since it allowed me to monitor everything in real time. However, even with this, the protocol, and everything else from Eupry, handling the mapping on our own turned out to be tough due to time and resources on our side, especially when dispatchers needed the trailers urgently. It took much longer than expected also because I had no baseline to work from; I was trying to keep the units running and disseminate the information. In other words, I was struggling to maintain operations while trying to carry out the validations, Mike O’Brien explains.
Therefore, I asked for Arsalan (Validation Lead at Eupry) to come assist. His expertise streamlined the entire process. He set everything up and conducted the necessary exercises, and everything became completely seamless.
Learn more about Eupry’s temperature mapping services.
3. The impact (and a continued partnership)
Since the first mappings, the Eupry team has carried out several mapping studies of Eagle Air Freight storage areas:
With this support, the mapping exercises are perfect, efficient, and exactly what we need to keep our operations compliant and effective. It has been a great partnership, and we are very happy that we partnered with Eupry,
Mike O’Brien says.
5 ways the services have affected Eagle Air Freight
The efficiency and reliability of the validation services have had several major impacts on Eagle Air Freight:
1. Making auditors happy
During the GDP certification process, Eagle Air Freight engaged an external firm to audit their compliance multiple times. On every occasion, their thermal validation and mapping procedures exceeded expectations.
We hired an outside firm to come in and conduct three different audits to prepare us for our GDP certification, and they were very impressed with all the mapping procedures that the Eupry team has helped us with, Mike O'Brien says.
2. Easing the transition into healthcare logistics
The efficiency of the services has streamlined Eagle Air Freight's transition into pharmaceutical logistics and continues to enhance this process as the company expands its fleet with new containers. Each addition requires validation, a task for which the company now has a seamless solution.
3. Setting Eagle Air Freight apart from competitors
This shift and solution also positioned the company as a premium service provider in a niche market that takes temperature compliance seriously.
With Eupry, we are always ahead of the curve when it comes to the quality of our temperature mappings, which has had a significant impact on our business. In Massachusetts, where the pharmaceutical sector is vast, there are very few carriers equipped to provide this level of service. Being able to provide this level of assurance to our clients sets us apart and has become a major selling point, distinguishing us from competitors, Mike O’Brien says.
4. Earning trust among clients
Although trust is a factor in almost every industry, it is more vital in pharma than most. As a result, when a logistics company works with pharmaceutical companies, there is very little room for error — another factor that underpins the importance of reliable temperature mapping and validation for Eagle Air Freight.
The stakes are much higher with life science logistics compared to general cargo. For general cargo, if something gets left behind, it is usually a minor issue — retrieve it and deliver it to the correct location. But with life science shipments, you only get one chance to get it right. Any mishap can lead to a loss of trust and credibility, which is incredibly difficult to recover,
says Mike O’Brien and continues:
We are starting to see that a lot of smaller carriers are entering the market by simply equipping a box truck with a temperature control unit and claiming they can transport pharmaceuticals. They might manage to offer lower rates and attract business initially, but this approach lacks the needed compliance, and eventually, manufacturers will demand proof of validated trailers and adherence to guidelines, especially when deviations occur. This is where the high-quality mapping and validation processes give us an edge. We are ahead of the curve and able to provide comprehensive reports and evidence of our legitimacy.
5. Enhancing service quality
Last but not least, the validations have allowed Eagle Air Freight to improve the quality of their offering.
This capability has also made it possible to improve quality in the transport of pharmaceutical materials. For instance, during a mapping session with Eupry, we identified hot spots in a specific trailer, allowing us to advise our clients on optimal cargo placement to minimize risk, which enhances our service quality, Mike O’Brien says.
Recap
Eagle Air Freight’s proactive approach in adopting stringent compliance measures and investing in reliable temperature compliance services with Eupry has set them apart in the healthcare logistics industry.
By focusing on healthcare logistics, they not only improved their service quality but also established themselves as a key player in a niche market, ensuring long-term growth and sustainability.

Temperature mapping solutions built for GDP and GMP - catalog
Discover how Eupry can enhance your logistics operations with advanced monitoring, mapping, and compliance solutions tailored to pharmaceutical logistics.
More case stories
Learn about how companies around the globe handle mapping, monitoring, and other compliance procedures with Eupry - or see how the solutions work in our product catalog.