Steam-in-place validation services
GMP-compliant service for pharmaceutical manufacturing
- Fast project delivery
- GMP-specialized validation engineers
- Cost-effective services

Trusted by 1000+ companies worldwide
What is steam-in-place (SIP) validation?
Steam-in-place validation proves that GMP production equipment – bioreactors, tanks, transfer lines, and other fixed installations – can be reliably sterilized using saturated steam without disassembly. The process verifies that steam reaches every surface at the required temperature and duration to achieve microbial kill.

The SIP validation challenge
Validation blocks production
SIP validation requires taking critical equipment out of production – meaning delays, disruptions, and lost revenue.
Complex systems are complex to validate
Proving steam reaches every location in piping systems with transfer lines, manifolds, and dead legs is complex and requires expertise.
Multiple vendors create coordination delays
Traditional SIP validation involves coordinating equipment, calibration, and consultants. Each handoff creates potential for errors and delays.
The steam-in-place validation solution
Eupry provides GMP-compliant SIP validation services for pharmaceutical and biotech manufacturers. We handle mapping, biological testing, and complete IQ/OQ/PQ for your equipment.
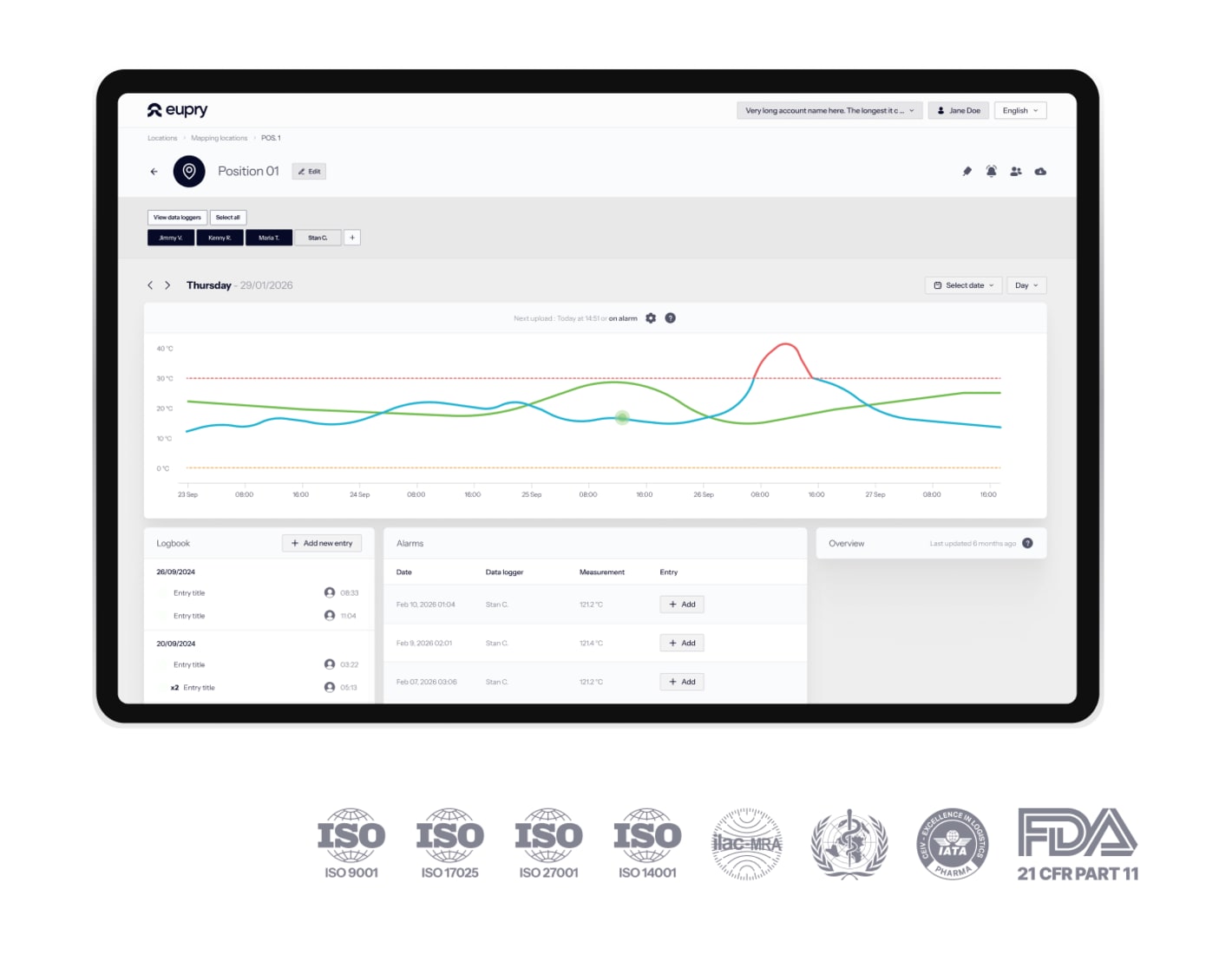
Complete validation service
Eupry handles the full validation from IQ through PQ. We place sensors, manage testing, perform F0 calculations, and deliver complete reports.
Validation in half the time
Our mapping kits and cloud-based software let us complete validation in half the time of traditional validation approaches.
Zero regulatory gaps
Proven protocols and GMP-specialized validation engineers ensures your validations are done right the first time - every time.
Complete IQ/OQ/PQ equipment validation
Installation qualification (IQ)
Verify correct installation per manufacturer specifications, confirm utility requirements (steam quality, pressure, flow), test safety systems, and document all instrumentation with ISO 17025 calibrated equipment.
Operational qualification (OQ)
Empty system validation with steam quality verification (dryness, non-condensable gases, superheat), multi-point mapping, pressure testing, and control system challenge under normal and edge conditions.
Performance qualification (PQ)
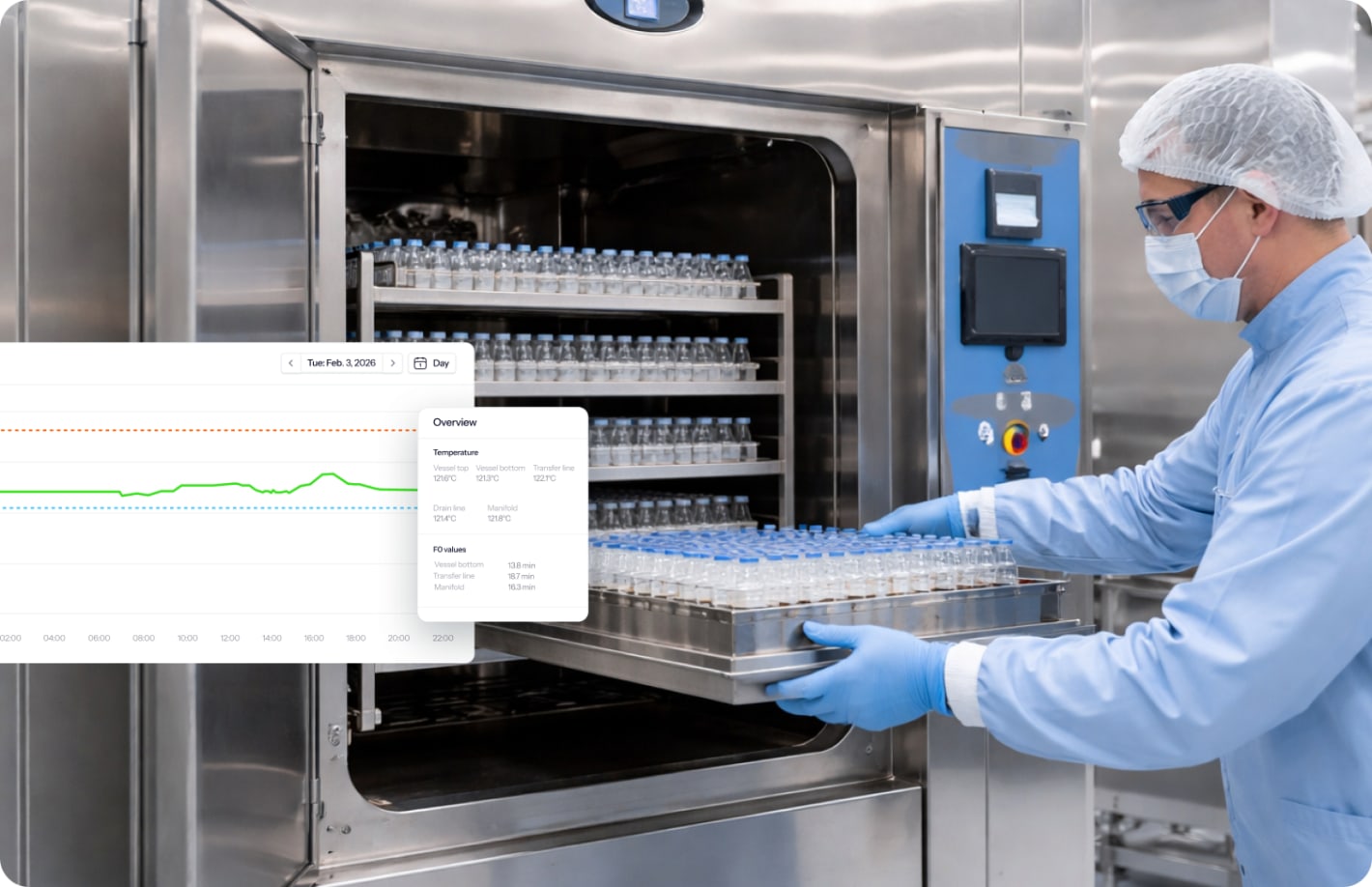
Loaded system validation including worst-case testing, heat penetration studies, biological indicators, and F0 verification (≥ 12 minutes at all locations).



Send us a message
Need more information or a tailored quote? Fill out the form, and we will be in touch as soon as possible.
Why choose Eupry for SIP validation?
Validation expertise
Our validation engineers have conducted hundreds of qualifications across pharmaceutical companies and understand the regulatory expectations from FDA, EMA, and MHRA inspectors.
Complete compliance
Every validation comes with ISO 17025 calibration with NIST traceability, ISO 17665 and EN 285 compliant protocols, 21 CFR Part 11-ready digital platform, and GMP Annex 15 aligned qualification approach.
Digital protocols
Our digital validation system captures every reading with timestamps and attribution, calibration certificates are embed, and you can generate complete audit reports in 3 clicks with full audit trails.
One solution for all
At Eupry, we handle validation for any temperature-controlled unit or facility in GMP and GDP – from bioreactors and tanks to autoclaves, warehouses, and cold rooms. One partner, all your compliance needs.
What we validate
- Bioreactors and fermenters: Single-use and stainless steel systems from benchtop to production scale, with temperature mapping, steam penetration verification, and transfer line validation.
- Tanks and vessels: Storage, buffer, mixing, and holding tanks for sterile production, verifying steam coverage to all internal surfaces and drainage adequacy.
- Transfer lines and manifolds: Piping systems connecting process equipment, with cold spot identification, dead leg sterilization verification, and steam trap validation.
- CIP/SIP systems: Integrated cleaning and sterilization systems, validating complete cycles from CIP through SIP for cleaning and sterilization effectiveness.
And any other controlled equipment and facility you need in GMP.
Get specific timeline and pricing information for your equipment.

Psst... We also validate autoclaves
(and any other GMP unit or facility)
Need autoclave validation for your laboratory or manufacturing sterilizers? We provide the same complete IQ/OQ/PQ services for steam autoclaves – from benchtop units to large production sterilizers.

Download a product catalog
Get an easy overview of all Eupry’s products from validation to calibration and automated monitoring temperatures, humidity, CO2 and more.
One vendor, all you need for thermal compliance
Collect validation, monitoring, and calibration in one solution.
- Up to 25% lower TCO
- One source of truth for all data
- 3-click audit reports
Get instant access to how the solutions work and the technical specifications.






