Steam sterilization validation services
Autoclave and SIP validation
Get your steam sterilization processes validated with Eupry's specialized services for autoclaves and steam-in-place systems.
- Complete IQ/OQ/PQ coverage
- Fast project delivery
- GxP-specialized validation engineers
- Cost-effective services
Trusted by 1000+ companies worldwide

Eurpy's steam sterilization validation services
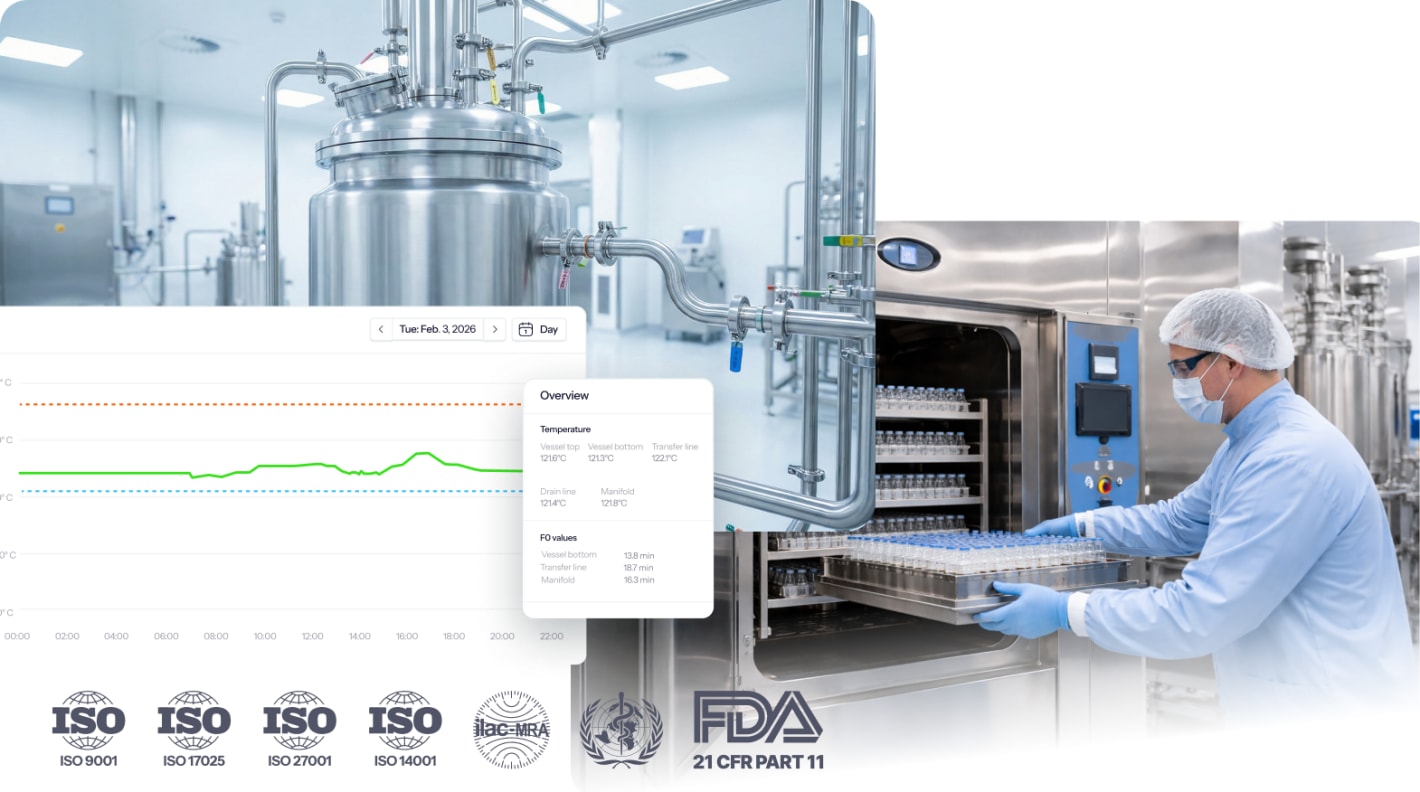
Eupry provides steam sterilization validation services for pharma manufacturing, validating both chamber-based autoclaves and fixed steam-in-place systems, covering all GxP requirements.
Types of steam sterilization validation services

Autoclave validation
Autoclave validation without the multi-vendor chaos, long timelines, or surprise costs.

Steam-in-place validation
GMP-compliant SIP validation service for pharmaceutical manufacturing.

And everything else you need
From validation to monitoring and calibration – one solution, one vendor, all you need for thermal compliance.
Why choose Eupry for pharma compliance?
Validation expertise
Our validation engineers have conducted hundreds of qualifications across pharmaceutical companies and understand the regulatory expectations from FDA, EMA, and MHRA inspectors.
Complete compliance
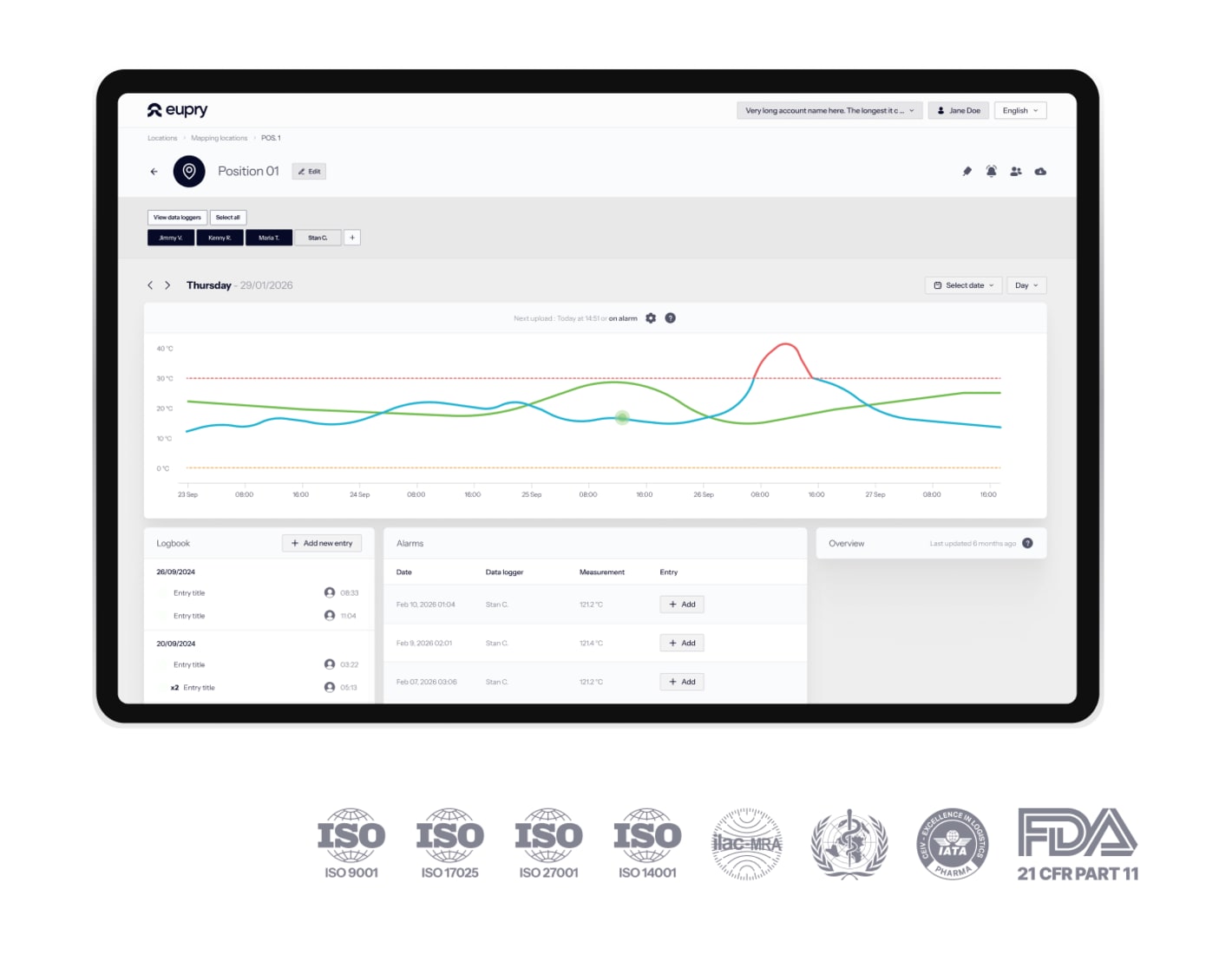
Every validation comes with ISO 17025 calibration with NIST traceability, ISO 17665 and EN 285 compliant protocols, 21 CFR Part 11-ready digital platform, and GMP Annex 15 aligned qualification approach.
Digital protocols
Our digital validation system captures every reading with timestamps and attribution, calibration certificates are embed, and you can generate complete audit reports in 3 clicks with full audit trails.
One solution for all
At Eupry, we handle validation for any temperature-controlled unit or facility in GMP and GDP – from bioreactors and tanks to autoclaves, warehouses, and cold rooms. One partner, all your compliance needs.

Download a product catalog
Get an easy overview of all Eupry’s products from validation to calibration and automated monitoring temperatures, humidity, CO2 and more.
Full validation of your...
- Autoclaves and steam sterilizers: Terminal sterilization of products, equipment, and materials across all cycle types.
- Bioreactors and fermenters: Steam-in-place validation for single-use and stainless steel systems.
- Tanks, vessels, and piping: Complete SIP validation for storage tanks, process vessels, and transfer lines.
And any other GMP equipment, unit, or facility.
Get specific timeline and pricing information for your equipment.

Send us a message
Need more information or a tailored quote? Fill out the form, and we will be in touch as soon as possible.
One vendor, all you need for thermal compliance
Collect validation, monitoring, and calibration in one solution.
- Up to 25% lower TCO
- One source of truth for all data
- 3-click audit reports
Get instant access to how the solutions work and the technical specifications.

What is steam sterilization validation?
Steam sterilization validation proves your sterilization process consistently achieves the required sterility assurance level (SAL) under defined conditions. For pharmaceutical manufacturers, this documented evidence is required by FDA, EMA, and GMP regulations to demonstrate your equipment reliably sterilizes products and materials.






