GxP-compliant temperature mapping solutions
Avoid delays, errors, and compliance risks with Eupry’s complete temperature mapping services and equipment.
- Audit-ready mapping with a click: Digital protocols, analysis, and GxP-compliant reports in the software.
- Validated right, every time: No costly errors or delays with expert guidance and live insights.
- Minimal disruptions: Fast and flexible setup keep the operational downtime to a minimum.
Get an instant overview of all service options, equipment specifications, and more.

Trusted by +1000 GxP-regulated companies worldwide
Traditional mapping slows you down (and risks compliance)
Temperature mapping is a regulatory must, but the traditional approach is often slow, disruptive, and risky.
Manual errors leading to risks
Errors, lost data, and costly re-testing. Paper-based processes create risk and waste resources.
Disruptions to your operation
Urgent re-mapping needs and lengthy on-site work pull staff away from core operations.
Disconnected processes and tools
Multiple systems and inconsistent methods create complexity, quality gaps, and extra costs.
Complex compliance demands
Full GxP compliance is critical, but challenging under shifting, complex, and often unclear standards.
Faster, more reliable mapping with specialized GxP solutions
in less time, at lower cost, with full confidence
With the fastest mapping solution you will find.
Digital mapping software
Create protocols, log data automatically, track studies live, analyze results, and generate GxP-compliant reports – all in one validation software.
Minimal disruptions
Fast setup and automated data transfer keep your operation running smoothly (plus, with continuous mapping, you can eliminate re-mapping).
Consistent, scalable process
Digital protocols and centralized data let you access results from anywhere, reuse templates, and roll out compliant studies consistently across sites.
Compliance confidence
Specialists, protocols, and equipment built for GxP – ensuring every study meets the latest standards and are tailored to your requirements, every time.
Compliance components
All your compliance boxes? Consider them ticked. Additionally, our specialists continuously update the service to adapt to evolving compliance standards, ensuring you remain fully compliant.
- FDA 21 CFR Part 11 compliance module
- ISO 17025-accredited calibration
- ISO/IEC 27001:2022-certified IT security
- ISO 9001-certified quality system
And much more.

Legal basis and guidance
The mapping solutions are designed with a legal basis in cGMP, USP <1079>, and EudraLex Volume 4 Part 1 Chapter 3, and based on guidance from WHO guidelines (supplement 8 and 7 and annex 8), DKD-R 5, FD X 15-140 (French standard), and the ISPE Standard “Controlled Temperature Chamber Mapping and Monitoring”.
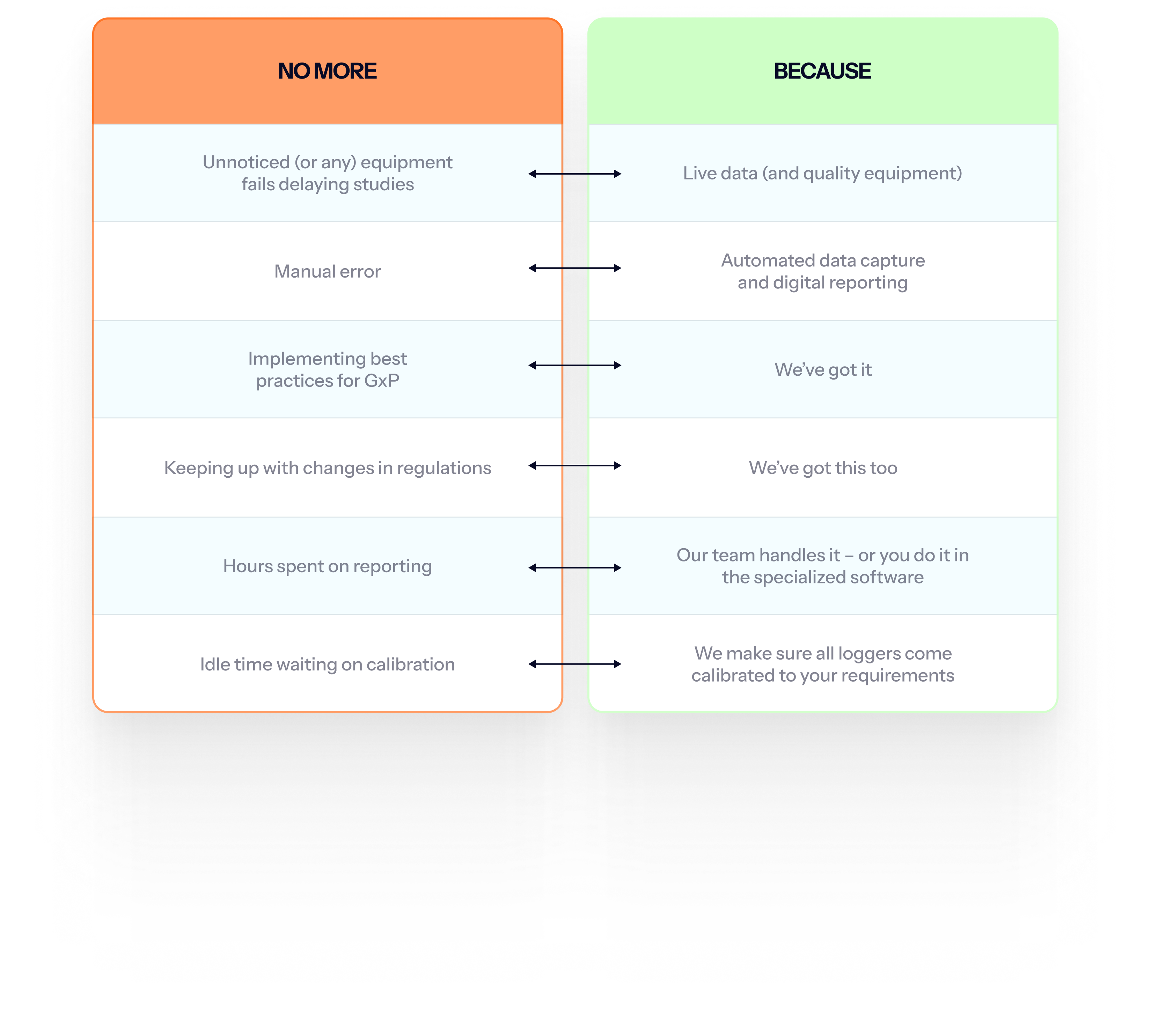
Flexible options to fit your needs
Pick a solution that fits your requirements (no more, no less) – from full on-site mapping services to rentable equipment. All options can be tailored to your needs and come with the same GxP-grade accuracy and compliance.
Choose for instance:
- Rentable mapping kit: You receive all the professional mapping equipment you need to perform the study yourself.
- Remote mapping service: We ship the equipment, guide your team through setup, and handle the analysis and reporting.
- On-site mapping service: Our validation specialists handle the entire study – from protocol to final report – on your premises.

Temperature mapping equipment built for GxP
All solution come with the same GxP-compliant equipment, ensuring fast, accurate, and compliant data for your studies.
- Wireless data loggers: Monitor conditions and spot issues instantly with automated, live monitoring.
- ISO 17025 or traceable calibration: All data loggers come calibrated to your standards.
- Specialized validation software: Digital (and centralized) planning, monitoring, and reporting of your validations.


Download your free mapping catalog
Reduce risks and secure a reliable, efficient result with efficient mapping services and equipment built for GxP. Learn more and find all the technical details in our product catalog.
Digital temperature mapping planning tool
How many data loggers do you need for your temperature mapping - and where to place them?
Our mapping planning tool does the calculations for you.
- Recommended logger quantity for full coverage
- Placement locations mapped to your unit dimensions
- Setup guidance aligned with the standard of your choice
See the tool in action
- and discuss your mapping project
Want to use the tool or discuss your specific mapping needs? Book a free session with one of our specialist.
Use the calendar to book a time slot that fits you. Or send us a message here.
Specialized GxP validation know-how
and mapping specialists on call (or on-site)
From validation to monitoring and calibration. Temperature compliance is what we do. With hundreds of mappings completed and deep knowledge of evolving GxP regulations, we can turn your complex requirements and operational needs into efficient, audit-ready results.


One vendor, all you need for thermal compliance
Eupry is more than mapping. It is one solution for all your thermal compliance: From mapping to monitoring and calibration – all in one GxP-compliant platform.
- Print audit reports in 3 clicks
- Track compliance for all sites on any screen
- One vendor, less complexity, lower costs
We have saved around 50-70% of the time we spent on temperature monitoring.
Anders Rasmussen, Senior Logistics Manager, Dechra Pharmaceuticals
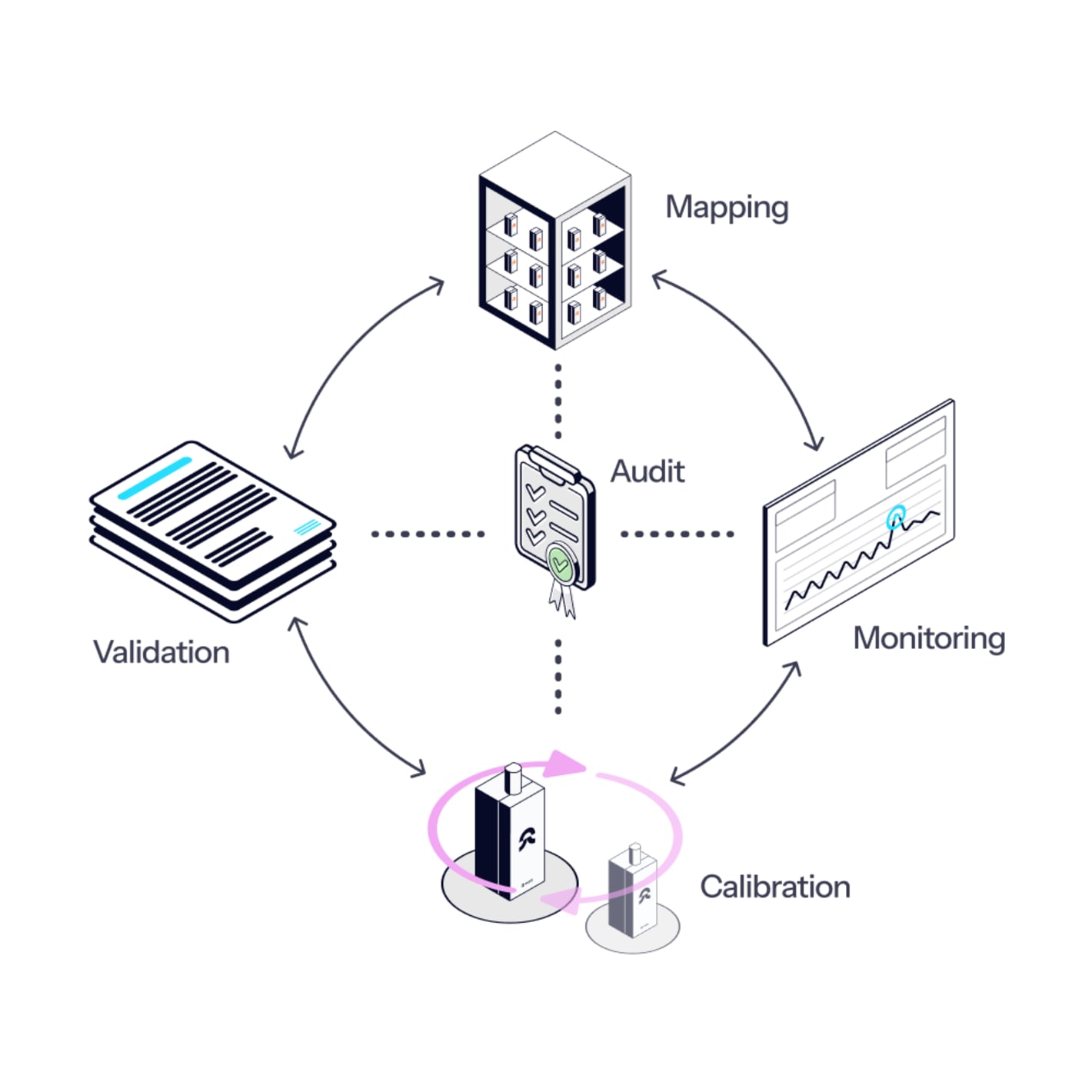
No calibration blockers
- Data loggers are always pre-calibrated, and certificates are linked in the mapping software.
- Choose to have the loggers calibrated in our ISO 17025-accredited lab or via traceable 3-point calibration.
- Plus, with Eupry's on-the-wall calibration solution, you can reduce the calibration time spend by up to 95%.

IQ and OQ of your unit – one provider for the full validation process
Our team can also manage your units' IQ and OQ process, saving you time, hassle, and cost.
- One provider for the full validation
- Cost- and time-optimized process
- Full GxP compliance confidence

What you get
Minimal downtime (and cost-efficiency)
Digitalization, validated processes, and live troubleshooting make your mapping faster and more cost-efficient than ever.
Compliance confidence (by minimizing risks)
Rest assured with live monitoring, GxP-tailored equipment, specialized guidance, and all the relevant ISO stamps.
Mapping expertise (when you need it)
No need to know the details of requirements for mapping various facilities and TCUs. Our specialists guide you through every step.
Simple scalability (with your operation)
From short-term equipment rental to full on-site service, our flexible approach lets you scale as new sites open or requirements change.
How the temperature mapping kit works
Perform your mappings internally using our GxP-compliant mapping kit with professional-grade equipment, specialized validation software, and access to specialist guidance - if you need it.
- Consult our specialists: To define your equipment need.
- Plan the study: Create digital test plans and logger placement.
- Set up in minutes: Place and connect the loggers with a click.
- Track the mapping: Act on issues right away with live monitoring.
- Create a digital report: Analyze data and generate digital reports.
- Return the kit: Once done, return the kit with the included label.
- We conduct exit calibration: You access certificates in the software.

How the full mapping service works
The ultimate solution if you need to meet requirements quickly. Our team of validation professionals handles every step of the mapping for you – planning, installation, and reporting.
- The project is scoped together with one of our specialists.
- Our team develops the protocol, and you review.
- Our specialists conduct the mapping on your site.
- Your results are analyzed, and you receive the final report.

How the remote mapping service works
The full mapping service also comes in a remote version. Our experts still take care of everything from protocol to the final report – you simply place the data loggers yourself.
Note: We tailor the solution to fit your SOPs and instructions. Do you prefer to install the equipment and leave the analysis and reporting to us? To conduct the mapping with our team as remote support? Have us design your protocol and then take it from there? We will find a way that works for you.
How DSV conduct GDP-compliant mapping with Eupry
Before DSV Sweden could utilize their new healthcare logistics center, they needed to ensure its ability to maintain temperature conditions in a way that was both swift and GDP compliant.

Client Testimonials
IT-validated mapping software
Full study overview
Data is automatically transferred from the wireless data loggers to the validation software where you can plan, track, and analyse your study.
The platform is IT-validated and accessed through a web app.
No downloads needed (= your IT department will love it).
- Complete overview of your mapping in real-time
- Digital reporting and audit-ready exports
- Access to data from anywhere in the world
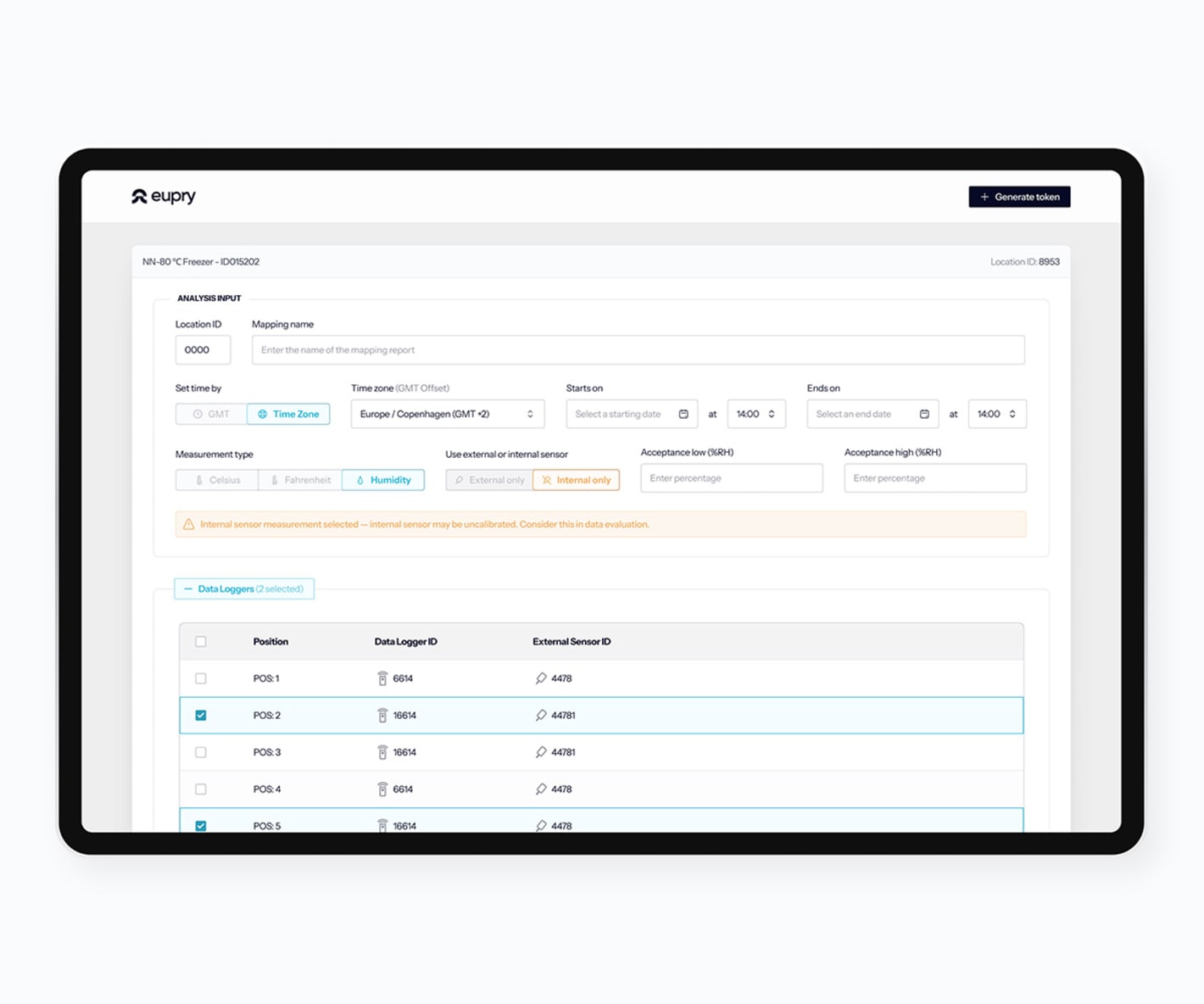
How does the mapping software work?
Start your mapping
Add facilities, review data logger info, and see calibration certificates.
Create test plans
Document training of your team, define data logger placement, etc.
Conduct your study
Run tests, add commenrs, and register non-conformities.
Analyze the findings
Analyze data, view graphs and box plots, and generate reports.
The sensors
The sensors work as easily-changeable calibration “antennas” that allow you to calibrate without switching devices – making it the most cost-effective calibration solution on the market.
Tip! Can’t find what you are looking for? These are just examples; see all options in the catalog.
The solution offers sensors for all temperature mapping requirements - here are some examples:

P1T - External Temperature Sensor
- Operating range: -50°C to +50°C (-58°F to 122°F)
- Resolution: 0.01°C (0.018°F)

P1TH - External Temperature & Humidity Sensor
- Operating range: 2°C to 50°C (35.6 °F to 122 °F)
- Resolution: 0.01°C (0.018°F) 0.01 %RH
- Humidity range: 20-80% RH (Non-condensing)
- Extended humidity range: 0-99% RH (Non-condensing)

P1CTH - External CO2, Temperature & Humidity Sensor
- Operating range: 2°C to 50°C (35.6 °F to 122 °F)
- Resolution: 0.01°C (0.018°F) 0.01 %RH
- Humidity range: 20-80% RH (Non-condensing)

P2T - External Temperature Probe
- Operating range: -90 °C to +50 °C (-130 °F to 122 °F).
- Resolution: 0.03 °C (0.054 °F)

P2TH - External Temperature & Humidity Probe
- Operating range: 2°C to 50°C (35.6 °F to 122 °F)
- Resolution: 0.01°C (0.018°F) 0.01 %RH
- Humidity range: 20-80% RH (Non-condensing)
- Extended humidity range: 0-99% RH (Non-condensing)

HC2A - High Precision Humidity Probe
- Accuracy @23 °C: ±0.5 %rh
- Application range: -50 to 100 °C
- Sensor element: HYGRIMER HT-1
- Long-term stability: <1 %rh per year with clean air
See all sensor and data logger options in the product catalog
See how the mapping solution works and find all product options in our free catalog.
- Technical specifications
- All sensor options
- Solution packages

Built for your industry
Our mapping solutions are designed to meet the developing needs of pharma, biotech, and healthcare logistics organizations.
How does Eupry's temperature mapping solution work for different TCUs and facilities?
From lab freezers to warehouses: No matter what, the custom solutions are tailored to your specific environments.
Note: The following are general recommendations – contact us for advice regarding your specific needs.
Examples of use cases for the mapping solutions:
Refrigerators, freezers and incubators
We recommend our standard data loggers calibrated to ISO 17025 for temperatures between -30 and +50°C. Typically, 9 or 15 loggers placed in a grid do the trick for a standard fridge.
Even small fluctuations in our freezer temperatures can have an impact on the quality of our samples. Eupry gives us trustworthy insights into this. This is essential in our work for making cancer vaccines real.
_Jennifer Bintz. Research Technician at InProTher
Ultra-low temperature (ULT) freezers
For the world of -80°C and below, our specialized external probes can be mounted through a service port or between the door and rubber gasket, thanks to their flat silicone cable making sure no unwanted openings are created eliminating deviations.
Typically, 15 loggers and sensors are sufficient. Each sensor comes with a 3-point calibration as per ISO 17025 standard, but additional calibration points can be added if needed.
Why it works:
- Specialized probes for extreme cold
- Easy mounting options through doors and lids
- ISO 17025-accredited calibration you can trust
Ambient warehouse storage
For ambient storage areas with temperatures ranging from 15°C to 25°C, we recommend our standard data loggers – usually with sensors calibrated to three points in our ISO 17025-accredited lab.
The number of data loggers needed varies based on your storage size. A small room might require 15 to 30 loggers, while a larger warehouse could need over 300. Our in-house quality department can assist in assessing floor plans and defining the exact number of sensors for your needs.
All data loggers follow a naming convention to make it easier for you to locate specific devices in larger areas.
Why it works:
- Tailored recommendations based on your facility
- Custom logger counts based on space
- Consistent naming for easy identification

Cold warehouse storage
Our standard data loggers with ISO 17025 accreditation are also ideal for colder storage areas. The method follows ambient warehouse though the temperature variance might be higher in certain areas of the warehouse causing the need for further data loggers.
The number of data loggers needed varies based on your storage size. Our in-house quality department can assist in assessing floor plans and defining the exact number of sensors for your needs.
All data loggers follow a naming convention to make it easier for you to locate specific devices in larger areas.
Why it works:
- Tailored recommendations based on your facility
- Custom logger counts based on space
- Consistent naming for easy identification

Storage containers and reefers
Containers pose unique challenges because of fluctuating outdoor conditions and the question of connection.
Utilize the same equipment as for warehouse storage but include consideration about changing outside conditions when determining amount and placement (we can help you here). Battery-driven 4G router fixes any issues with connectivity.
The number of data loggers needed varies based on your storage size. Our in-house quality department can assist in assessing floor plans and defining the exact number of sensors for your needs.
Why it works:
- Durable for on-the-road conditions
- Tailored recommendations based on your facility
- Custom logger counts based on space

Want more details?
Let us talk. Book a 15-minute session to talk about how you can best utilize the temperature mapping solutions for your needs.
Need a quote or more information? We are here to help. Temperature monitoring and compliance is what we do, and we know how to turn your requirements into action and can help uncover your needs; from quick questions to complex projects.
Let us know what you are looking for, and we will send the information – or set up a talk.
Would you rather explore on your own? It is your lucky day. Download our solution catalog.
Eupry’s temperature mapping services
Here are the answers to some of the most frequent questions we get about our temperature mapping solution.
Get started - or learn more
See your options and technical specifications in our catalog – or send us a message to get answer or a tailored price for your project.









