Webinars
Watch live and on-demand webinars about everything from hands-on mapping guidelines to automating your compliance practices.
Upcoming webinars

Mastering Temperature Mapping
February 19th, 2026
The complete mapping textbook launches in February 2026. Join the author for a walkthrough - and get a free copy of the book*.

How to pass FDA inspections
March 19th, 2026
How do you make sure your monitoring, mapping, and similar compliance processes are 21 CRF Part 11-compliant? Lean how to turn 21 CFR Part 11 into simple steps.

Eupry Q1 2026 product update
March 31st, 2026
Join Eupry's Q1 2026 product launch webinar to get an update on the latest technology in modern GxP compliance.
Stay up to date on upcoming webinars
Get informed about new webinars, workshops, and similar resources directly in your inbox.
On-demand sessions

AI in GxP today: What's working and what's risky?
The FDA published a discussion paper, and the EU is working on regulations. Is AI going to be a game-changer in GxP?
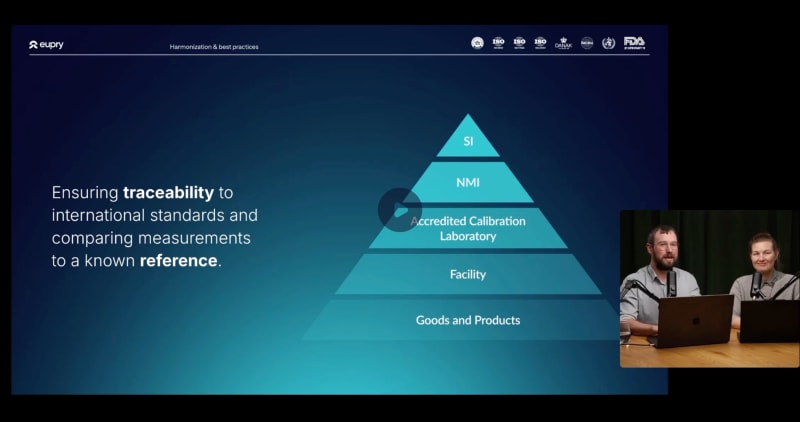
Overcoming calibration challenges in GxP
Calibration can be a labor-heavy and risk-filled process. Join us for a session where we explore the main challenges of calibration in GxP and the practical steps to overcome them.
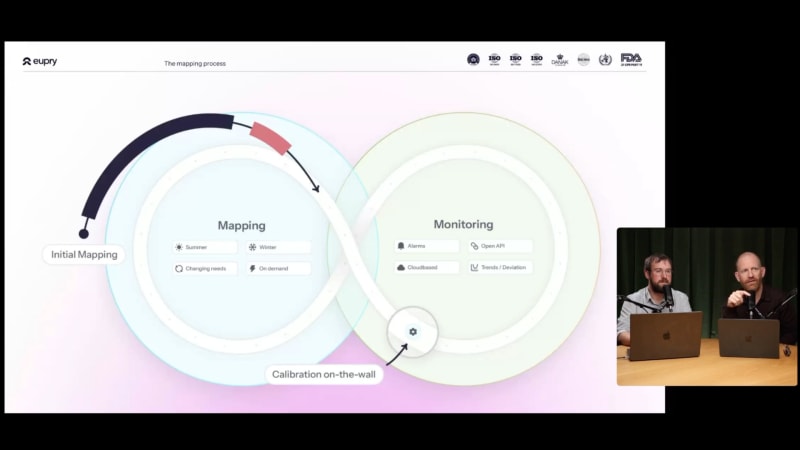
How to harmonize temperature validation (and mapping)
Same validation process everywhere. Full compliance. Sounds impossible? It is not – here is how.
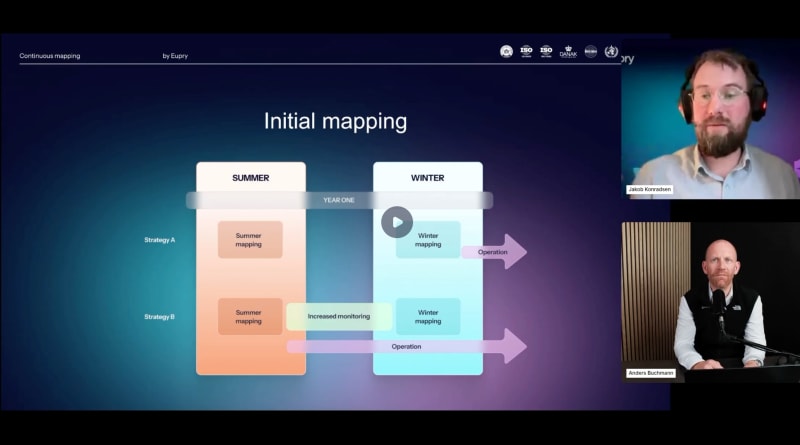
How to conduct winter temperature mapping in GxP
Understand the best practices for seasonal temperature mapping – including when it’s required, how to prepare, and what regulators expect.

VIRTUAL temperature mapping - how and why?
Cut months off your validation timelines and cut your mapping workloads - without sacrificing compliance. Everything you need to know about the virtual mapping framework.

Guidelines for continuous temperature mapping = no more re-mapping
Is CONTINUOUS temperature mapping going to change how we handle temperature compliance in pharma logistics and similar GxP sectors? We think so. Learn why - and how it works.
Temperature mapping in GxP
From planning your study to data analysis, efficient reporting, and the errors you could avoid, understand the practices for performing reliable, risk-based mapping that meet WHO and ISPE standards.

Audit-ready temperature compliance and documentation
Audit preparation can be a straining, worryful, and extremely time-consuming process – but it doesn’t have to be. Learn how to proactively prepare your mapping, monitoring, and related processes to meet auditor expectations in GxP.
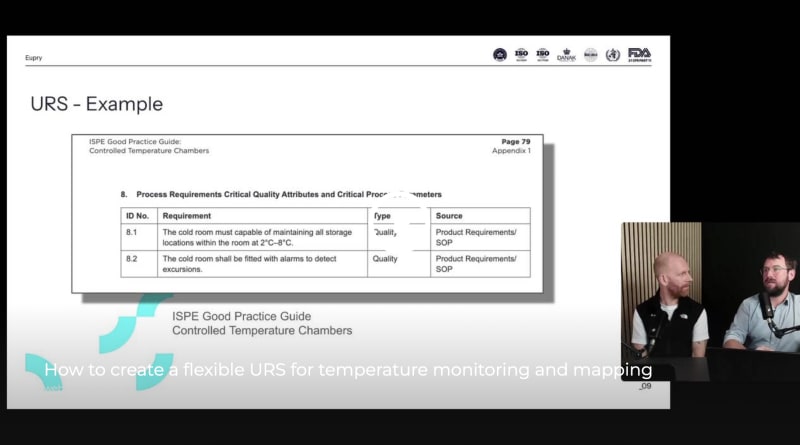
How to create a flexible URS for monitoring & mapping
Your URS shapes your compliance. So, how do you design a lasting, efficient URS that leads to lasting, efficient compliance – aligned with WHO and ISPE guidelines?

Guidelines for performing a summer mapping in GxP
Learn how to determine if seasonal mapping is a need-to-have for you, the best practices for conducting temperature mapping during summer, and common pitfalls to avoid.

Temperature mapping in GxP - data logger placement and amount
Learn the best practices for determining how many data loggers you need – and where you should place them – for your next temperature mapping study.
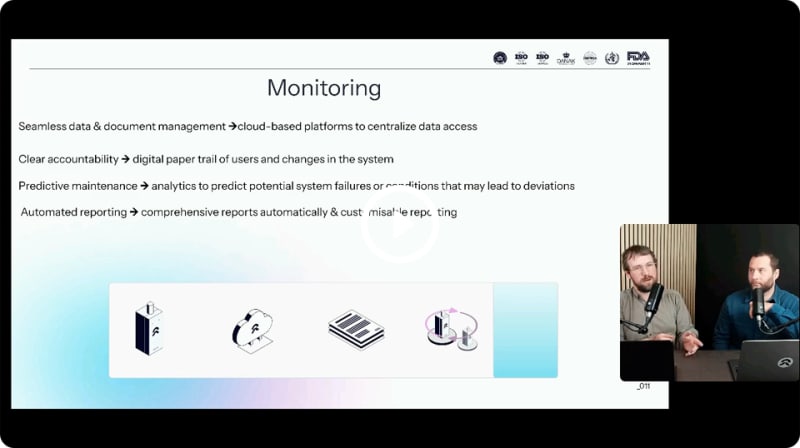
Automated temperature mapping and monitoring
Best practices and mistakes to avoid. No more manual work. Is automation changing how we handle temperature mapping in GxP?
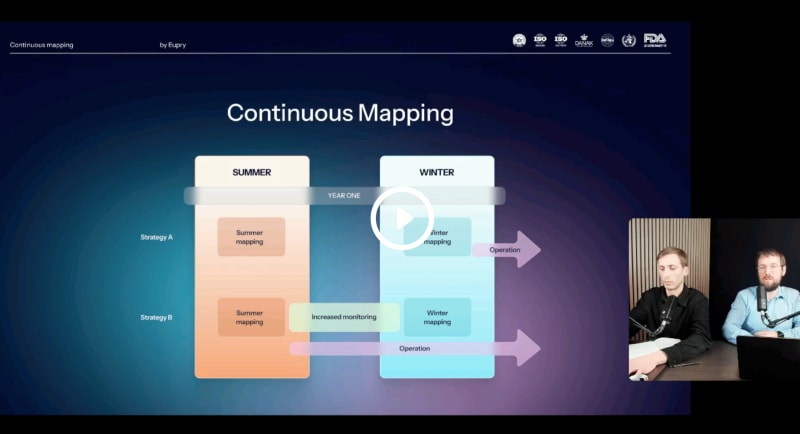
Standardize GDP validation – and eliminate re-mappings
Learn the guidelines for GDP-compliant validation and the continuous mapping framework to ensure compliance – without the need for re-validation.
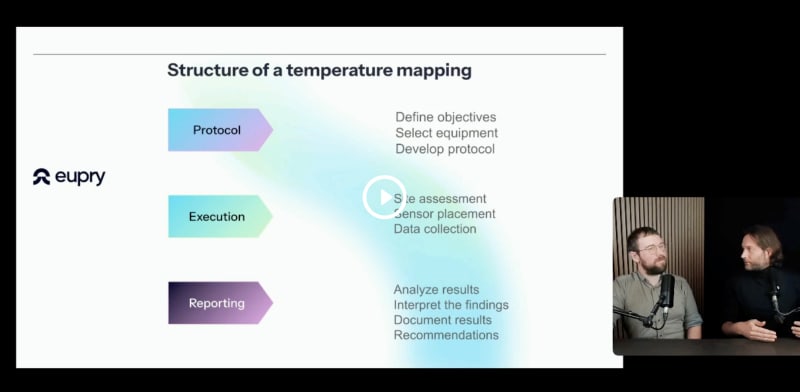
9 mistakes for thermal mapping
What are the most common errors at each stage of a mapping, and how can you mitigate the risk of them happening to you?
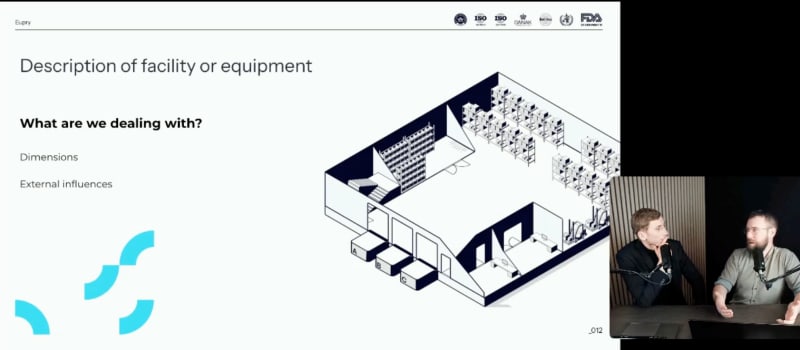
Designing GxP-compliant mapping protocols
Uncover the best practices (and most common pitfalls) for developing temperature mapping protocols for the pharmaceutical sector and other highly regulated industries.
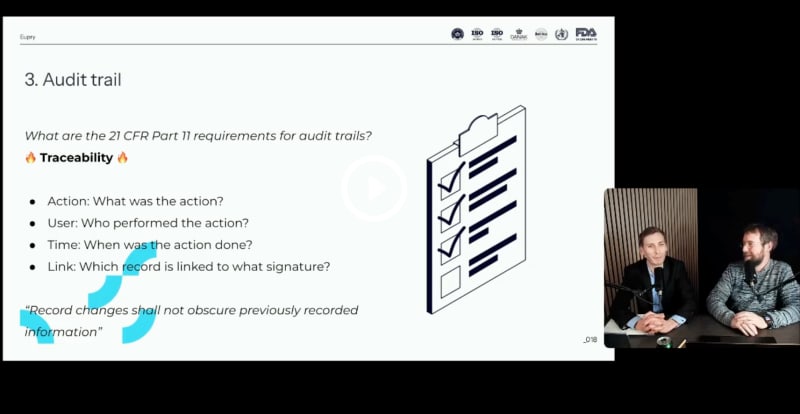
21 CFR Part 11-ready monitoring & mapping
Join us for a step-by-step guide to keeping your temperature compliance processes and records up to 21 CFR Part 11 requirements.

Winter temperature mapping - considerations and best practices
Is winter mapping needed for your operations? What are the special conditions you should consider? And what do guidelines from WHO, FDA, GxP, and other regulatory bodies have to say about the subject?

GACP/GMP compliance in cannabis
Learn the ins and outs of GACP and GMP in the cannabis industry to ensure your operation complies with temperature and humidity regulations.

Risk-based temperature mapping: Aligning with industry standards and guidelines
Gain a better understanding of how to apply a risk-based approach to your temperature mapping studies within life-science, biotech, logistics and other highly regulated fields.

The 6 essential steps to navigating the FDA’s adoption of GDP guidelines
Learn how to understand the differences and overlap of the FDA's and GDP guidelines for temperature compliance to successfully operate within an international supply chain.

How to navigate WHO’s temperature mapping guidelines
Understand and navigate the WHO's guidelines for temperature mapping — and how to turn them into actionable plans.

5 components to performing a GxP-compliant summer mapping
Understand the intricacies of performing a temperature mapping by accommodating to the unique conditions of summer.

Utilizing digital tools to mitigate risks in your temperature compliance processes
Learn how utilizing digital tools can reduce risks, eliminate manuel work, and reduce (unnecessary) time spent on temperature monitoring, mapping, and calibration.
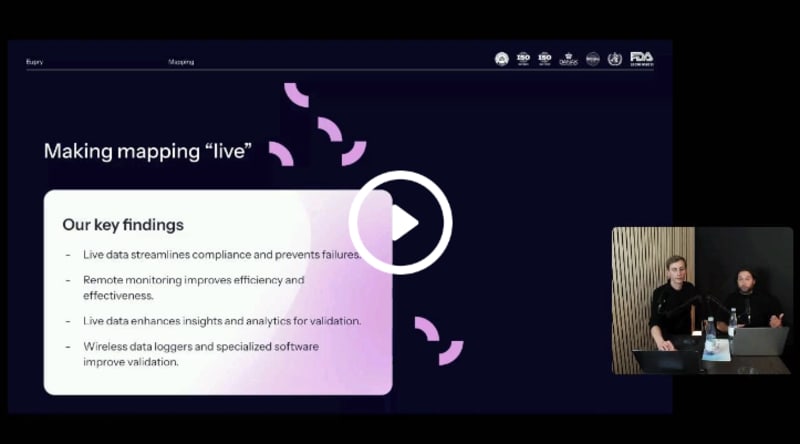
How to eliminate the gaps in temperature compliance
Siloed processes can lead to errors, wasted resources, and valuable information being lost. Learn how to unify both monitoring, calibration and mapping to streamline your temperature compliance.

The hidden costs of temperature compliance
Do you struggle to identify the hidden costs of temperature compliance? Dive into how to pinpoint ALL costs of temperature monitoring, mapping, and calibration — and the steps to eliminating unnecessary costs.

How to create a GxP compliant mapping report
Gain insights into the making the best GxP compliant temperature mapping report, to save (a lot of) time and confusion on the landscape of the regulatory requirements.

5 steps to prepare for a temperature compliance audit
Are you sick of the frustrating mess that (often) is audit preparation? Learn practical steps to efficiently prepare for your temperature compliance audits.

Guidelines for automating your temperature compliance
How to remove the mess from temperature monitoring, mapping, and data logger calibration through automation – and the pitfalls to avoid.

Utilize your temperature mapping data to improve compliance and efficiency
Going from data to action – are you making the most of your temperature mapping reports? Learn how to get the most out of the data from your mappings.

Overcoming data logger calibration challenges in GxP environments
Dive into which calibration elements you need to be audit-ready, how to choose calibration points, and streamline the process across your organization.

Understanding 21 CFR part 11 guidelines for temperature records
How do you comply FDA 21 CFR PART 11 for temperature records, in this webinar we will give you a structured approach and some examples.
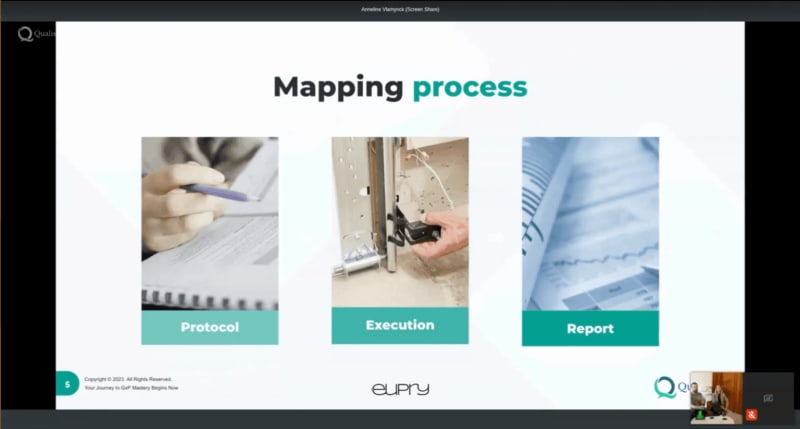
The 14 elements of a top-notch mapping protocol for GMP and GDP
Get insights into the fundamental elements of a temperature mapping protocol that can save you both time, cost, and frustration.

9 things that go wrong in temperature mappings
Dive into the most common mistakes happening during thermal mappings – and learn how you can avoid them.